Merck Ltd. and the government-funded Development Center for Biotechnology (DCB) jointly announced yesterday a cooperation agreement to establish an Asia Technology Training Center (ATTC) in Taiwan.
Merck will invest approximately NT$10 million (US$308,000) in the training center, bringing its own systems and plans to have two to three lab technicians in addition to a technical manager, who will be responsible for coordinating clients throughout Asia, said William Chen (
"The initial investment is small, but the potential return on capital is exponential," he said.
Since DCB will be providing a majority of the talent and support, Merck will have a smaller staff, Chen said.
"The key is our technical manager, who will have to act as coordinator for clients across Asia and rely on our expertise in scale production of protein-based drugs and consulting services," Chen said.
"The ATTC is not merely a training center; it is where clients can come and use our expertise and perhaps inquire about technology transfer," he said.
German-based Merck established its Taiwan branch in 1989 and distributes laboratory equipment and raw ingredients for research in sciences. The company has two training centers in the world -- one in Germany and another in the US. ATTC will be the third facility and the only one in Asia.
Under the agreement, Merck will set up ATTC on the DCB campus in Sijhih City (汐止), Taipei County. DCB will provide the space, facilities, consultants and supplies, while Merck will share its cooperation model with customers and partner DCB in its global R&D efforts.
The competition to house this facility was high, with Japan, China, India and South Korea all vying for the opportunity to enhance their biotechnology sector, DCB officials said.
"DCB is quite pleased to have won Merck's approval. The joint proposal drawn up by Merck Taiwan and DCB was done within a week and beat out proposals from other Asian countries -- some of which had been working on the proposals for a year," said Huang Bor-fuei (
DCB and Merck Taiwan were able to edge out the competition as their proposal went beyond the simple concept of a "training center" and incorporated more business integration opportunities, Huang said.
Taiwan has an advantage in this sector in Asia because of its clear rules on intellectual property rights protection, DCB's well-established bioprocessing team and R&D facilities, and a current good manufacturing practice, or CGMP, pilot plant to support large-scale production, he said.
DCB chairman Wu Shuh-min (
Wu was referring to the legislature's approval of the Biotech and New Pharmaceutical Development Act (
"It also shows that Merck recognizes the efforts DCB has made in 2007 on the international stage, striking cooperation deals with Boehringer Ingelheim of Germany, Crucell of Netherlands and MediVas of the United States," Wu said
Looking ahead, DCB, and by corollary, Taiwan's biotechnology industry, will benefit from more international exposure and recognition, and smaller biotech firms will gain access to world-class consulting and services in protein-based drug production, Huang said.
One of several Taiwanese biotechnology firms present at yesterday's event said it had learned more about potential opportunities to cooperate with DCB.
“The case put forward by DCB looks good, and if costs are acceptable we will probably seek to cooperate with them. The services that Merck and DCB provide certainly helps the process of protein-based drug production,” said
Cheng Li-ting (鄭力廷), a researcher at Sijhih-based Yeastern Biotech Co Ltd (益生生技).
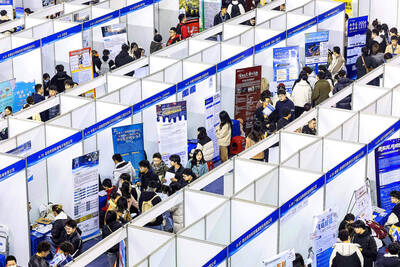
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
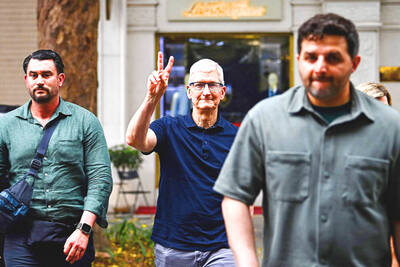
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

Taiwan Transport and Storage Corp (TTS, 台灣通運倉儲) yesterday unveiled its first electric tractor unit — manufactured by Volvo Trucks — in a ceremony in Taipei, and said the unit would soon be used to transport cement produced by Taiwan Cement Corp (TCC, 台灣水泥). Both TTS and TCC belong to TCC International Holdings Ltd (台泥國際集團). With the electric tractor unit, the Taipei-based cement firm would become the first in Taiwan to use electric vehicles to transport construction materials. TTS chairman Koo Kung-yi (辜公怡), Volvo Trucks vice president of sales and marketing Johan Selven, TCC president Roman Cheng (程耀輝) and Taikoo Motors Group

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last