Drugmaker Merck & Co. will donate its new vaccine against rotavirus, a highly contagious diarrheal illness, to Nicaragua under a program that will inoculate all newborns in the Central American country for free for three years.
The program is part of a strategy by Merck, one of the few major pharmaceutical companies still making vaccines, to run demonstration projects in poor countries showing the public health benefits of vaccines as a way to increase their use in developing countries.
The US$75 million gift will provide each of three doses of Merck's RotaTeq vaccine to all of the approximately 150,000 children born in Nicaragua for the next three years. After that, New-Jersey-based Merck will provide the vaccine to the Nicaraguan Ministry of Health at a dramatically reduced price, Margaret McGlynn, president of Merck's vaccines division said.
The project was announced on Friday morning at the Clinton Global Initiative, the former president's second summit aimed at getting business, political and charity leaders to provide aid on poverty, health care and other issues in developing countries.
The grant will cover training for doctors and nurses in Nicaragua, along with technical assistance on storage of the vaccine, which must be kept refrigerated, and on collecting data on vaccine effectiveness and any side effects.
"We chose Nicaragua because they have demonstrated a strong commitment to protecting the health and well-being of their population, particularly their children," and have a strong nationwide vaccination program, McGlynn said.
Rotavirus infections are transmitted by contact with feces and cause gastroenteritis, with symptoms of severe diarrhea, fever and vomiting.
"Virtually every infant ends up getting this disease," McGlynn said.
Worldwide, the virus causes about 2 million hospitalizations and 600,000 deaths each year among children younger than five. Most of the deaths occur in poor countries with higher rates of malnutrition and limited medical care. Even in tiny Nicaragua, rotavirus caused dozens of deaths and thousands of illnesses last year.
RotaTeq was launched in the US in February right after it was approved by the US Food and Drug Administration. In patient studies, it prevented 74 percent of cases of gastroenteritis and 98 percent of severe cases caused by four common strains of rotavirus. Some public health officials have said that Merck should have done more of its testing in poor countries.
Merck, which is currently seeking approval to sell RotaTeq in more than 100 countries, plans to do clinical testing in Africa and Asia.
The project in Nicaragua will provide additional data on the vaccine's effectiveness and safety in developing countries.
The vaccine schedule calls for giving three doses of RotaTeq -- a liquid sprayed through a tube into the mouth rather than given by injection -- between six weeks and six months after birth.
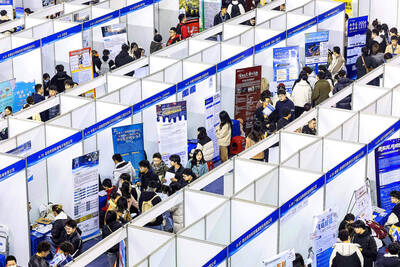
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see
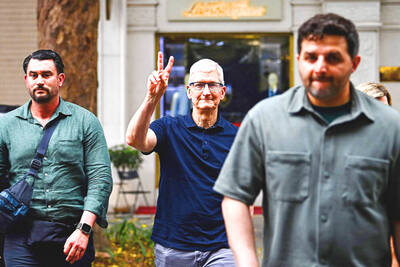
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last

US CONSCULTANT: The US Department of Commerce’s Ursula Burns is a rarely seen US government consultant to be put forward to sit on the board, nominated as an independent director Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday nominated 10 candidates for its new board of directors, including Ursula Burns from the US Department of Commerce. It is rare that TSMC has nominated a US government consultant to sit on its board. Burns was nominated as one of seven independent directors. She is vice chair of the department’s Advisory Council on Supply Chain Competitiveness. Burns is to stand for election at TSMC’s annual shareholders’ meeting on June 4 along with the rest of the candidates. TSMC chairman Mark Liu (劉德音) was not on the list after in December last