For the obsessively guarded, conservatively dressed and unflamboyant Oeri, Hoffman and Sacher families, avian flu could be good news. Over the next two years, the heirs of Fritz Hoffman, founders of Roche, one of the world's most powerful pharmaceutical companies, and who already rank as among the world's richest families, could see their combined ?10 billion (US$17.7 billion) fortune reach giddy heights.
Twenty members of the founding family control Roche, which industry analysts estimate will benefit from the Tamiflu drug thought to relieve the symptoms of avian flu, with extra profits of ?500 million this year and ?1 billion next.
And since the family owns about 10 percent of shares and crucially 50.01 percent of voting rights, they will ensure that no outside interests seize their company and enjoy the profits -- though many would like to.

PHOTO: NY TIMES NEWS AGENCY
As avian flu spreads from southeast Asia into Europe, sparking fears of a worldwide epidemic that medical experts say could claim 50 million lives, Roche, famous as the company behind the Valium tranquillizer, appears poised to clean up.
The Basel-based company is already the fastest growing drugs firm in the world with a share performance to match. Investor returns have increased 50 percent in a year. Last week its share price reached record highs after it said third-quarter profits rose by 20 percent to ?3.9 billion.
And that growth is primarily due to a drug it did not even invent. It was US biotech firm Gilead that developed Tamiflu. But nine years ago, Gilead signed a development and licensing agreement with Roche.
It is currently the subject of legal action which will be resolved in a year. Gilead claims Roche has been negligent in its manufacture of Tamiflu which has led, Gilead says, to a series of product recalls. Gilead also says Roche has failed to market the product well, which has reduced the potential revenue the drug could have made. Roche categorically refutes the allegations.
But as legal action rumbles, Roche faces other possibly more serious threats. The firm is under unrelenting pressure to increase production of Tamiflu. In the US, Senator Charles Schumer has threatened legislation compulsory to license Tamiflu unless Roche allowed generic producers to boost the number of pills in circulation.
The senior Democratic senator accused Roche of "putting profits ahead of world safety." He has threatened, along with Republican lawmakers, to introduce legislation to force Roche to relax its stranglehold on the drug.
Pressure appears to have paid off. Last Thursday, after a month of holding its position, Roche said it would talk to four generic drug manufacturers about increasing production. Health campaigners say this is no guarantee that Roche will act. If it does, it will delay matters as long as possible so it gets the most revenue possible before low-cost manufacturers get in on the act.
Michael Bailey, of campaigning group Oxfam says: "This situation is absurd. A government will have to make a move because Roche seemingly can't deliver. It's a classic case of international intellectual property law not working."
"It seems Roche is holding on as long as possible before allowing generic companies the right to produce so it can make as much cash as possible," he says.
Roche says it can produce 10 million treatments each year and so far 40 governments have placed orders for Tamiflu. It says that although any government ordering a bulk supply should expect to wait a year before its order is satisfied, generic firms have to prove that they can safely manufacture Tamiflu as there are 10 complicated steps involved in its production.
While Roche is coming under intense scrutiny, the financial community is hugely supportive of the company. After all, today it is possibly Europe's best performing drug firm thanks to its investment in biotechnology companies way before other drug majors.
Much of its profits are due to its 55 percent stake in Genentech, the US biotechnology company behind targeted cancer drugs such as MabThera and Avastin. The company has strengthened its diagnostic division and is leading moves to tailor drugs to individual patients through the use of new technologies.
Denise Anderson, head of healthcare at Kepler Equities, says: "They have made paradigm changing moves. They think more carefully about trends. They just started talking about healthcare IT. Right now they are in a sweetspot where successful drugs are coming on stream and I expect that to last for a good few years."
Roche has resolutely rejected merger offers. Novartis, its bigger rival, also from Switzerland, bought a near third share in the company four years ago. It has long been thought that this was a prelude to a merger that would produce a national Swiss champion.
But the three family clans believe mergers yield no value and divert management's energy away from day-to-day business.
Though it is Fritz Gerber and Andre Hoffman who sit on Roche's board and look after the interests of the families, Roche is run by Franz Humer, who earns ?5.4 million -- the third biggest basic salary of any publicly quoted executive in Europe.
"The Hoffman family don't need money," says an informed insider. "For them it's a prestige thing. They are typically Swiss. They are conservative and do things for the long term."
But others believe that a new generation of Roche heirs will not block a merger, as the need to create a buttress against US giant Pfizer and UK rival GlaxoSmithKline deepens.
While the pharmaceuticals industry emerged in Europe with aspirin being discovered at Bayer in Germany, its center of gravity has shifted to the US, attracted by its research scientists and lack of price controls.
But Switzerland has a reserve of scientific expertise -- Swiss drug research papers receive more citations even than US ones.
Although its expertise is not disputed, the firm has been no stranger to controversy. In the 1970s, Stanley Adams, a Roche employee, handed over documents to the European Economic Community (EEC) as it was then, detailing how the company kept the price of vitamins high with the explicit collusion of its supposed rivals. But an EEC bungle identified Adams. Roche decided to prosecute and he was imprisoned under tough Swiss commercial secrecy laws. His wife then committed suicide. Twenty years later, Roche was at it again -- marshalling a price-fixing cartel in exactly the same product. It was fined more than US$500 million by US and EU competition regulators.
The firm has also been singled out by health campaigners for dragging its feet in allowing AIDS-devastated countries in Africa to get access to vital medicines. It was one of the 39 companies that threatened to take South Africa to court to overturn its right to produce cheap copies of expensive brand-name anti-HIV drugs.
And today though it is unclear how well Roche is helping the world prepare for what could be a devastating flu epidemic.
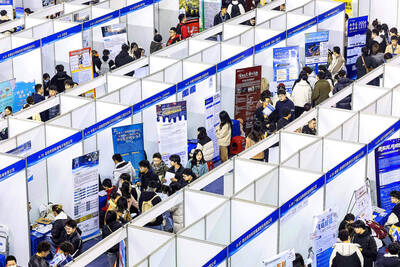
Stephen Garrett, a 27-year-old graduate student, always thought he would study in China, but first the country’s restrictive COVID-19 policies made it nearly impossible and now he has other concerns. The cost is one deterrent, but Garrett is more worried about restrictions on academic freedom and the personal risk of being stranded in China. He is not alone. Only about 700 American students are studying at Chinese universities, down from a peak of nearly 25,000 a decade ago, while there are nearly 300,000 Chinese students at US schools. Some young Americans are discouraged from investing their time in China by what they see

Taiwan Semiconductor Manufacturing Co (TSMC, 台積電), the world’s largest contract chipmaker, yesterday reported record sales for the first quarter, which analysts attributed to solid demand for emerging technologies. Consolidated revenue totaled NT$592.64 billion (US$18.51 billion) in the January-to-March period, up 16.5 percent from a year earlier, but down 5.26 percent from the previous quarter, TSMC said in a statement. The first-quarter revenue beat analysts’ average projection of NT$579.5 billion, Bloomberg News reported. That performance lends weight to expectations that the world’s most valuable chipmaker would return to solid growth this year after weathering a post-COVID-19-pandemic cratering of smartphone and computer sales. TSMC is budgeting
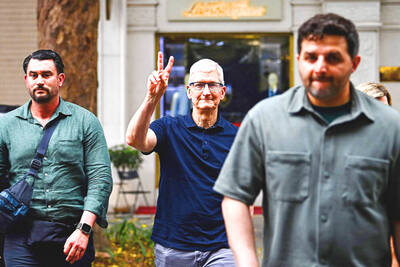
MAJOR DROP: CEO Tim Cook, who is visiting Hanoi, pledged the firm was committed to Vietnam after its smartphone shipments declined 9.6% annually in the first quarter Apple Inc yesterday said it would increase spending on suppliers in Vietnam, a key production hub, as CEO Tim Cook arrived in the country for a two-day visit. The iPhone maker announced the news in a statement on its Web site, but gave no details of how much it would spend or where the money would go. Cook is expected to meet programmers, content creators and students during his visit, online newspaper VnExpress reported. The visit comes as US President Joe Biden’s administration seeks to ramp up Vietnam’s role in the global tech supply chain to reduce the US’ dependence on China. Images on

New apartments in Taiwan’s major cities are getting smaller, while old apartments are increasingly occupied by older people, many of whom live alone, government data showed. The phenomenon has to do with sharpening unaffordable property prices and an aging population, property brokers said. Apartments with one bedroom that are two years old or older have gained a noticeable presence in the nation’s six special municipalities as well as Hsinchu county and city in the past five years, Evertrust Rehouse Co (永慶房產集團) found, citing data from the government’s real-price transaction platform. In Taipei, apartments with one bedroom accounted for 19 percent of deals last